
Thomas Krüger, Marc Amkreutz, Peter Schiffels, Bernhard Schneider, Otto-Diedrich Hennemann, and, Thomas Frauenheim.The Journal of Physical Chemistry C 2007, 111 DFT Periodic Study of the Adsorption of Glycine on the Anhydrous and Hydroxylated (0001) Surfaces of α-Alumina. Corinne Arrouvel, Boubakar Diawara, Dominique Costa, and, Philippe Marcus.The Journal of Physical Chemistry C 2008, 112 Interface Dipoles Observed after Adsorption of Model Compounds on Iron Oxide Films: Effect of Organic Functionality and Oxide Surface Chemistry. Jan Wielant, Ralf Posner, Guido Grundmeier and Herman Terryn.Study of the Self-Assembling of n-Octylphosphonic Acid Layers on Aluminum Oxide. Tom Hauffman, Orlin Blajiev, Johan Snauwaert, Chris van Haesendonck, Annick Hubin and Herman Terryn.Interface Chemistry and Molecular Interactions of Phosphonic Acid Self-Assembled Monolayers on Oxyhydroxide-Covered Aluminum in Humid Environments. Michael Maxisch, Peter Thissen, Miroslaw Giza, and Guido Grundmeier.The Journal of Physical Chemistry C 2011, 115 Molecular Interactions of Electroadsorbed Carboxylic Acid and Succinic Anhydride Monomers on Zinc Surfaces. Ordered Nanostructures on a Hydroxylated Aluminum Surface through the Self-Assembly of Fatty Acids. Asadauskas, Jessem Landoulsi, and Jean-François Lambert. Irma Liascukiene, Nesrine Aissaoui, Svajus J.The Journal of Physical Chemistry C 2016, 120 Effect of Anodic Aluminum Oxide Chemistry on Adhesive Bonding of Epoxy.

The Journal of Physical Chemistry C 2020, 124 Surface Reconstruction, Hydration, and Adhesion of Epoxy to the (0001) Surface of α-Berlinite: Insights from Density Functional Theory Calculations. Fiber Implantation for Interfacial Joining of Polymer to Metal. Surface Structure Controls Self-Metalation: In-Situ IR Studies of Anchored Porphyrins on Atomically-Defined Cobalt Oxide Surfaces. Tobias Wähler, Ralf Schuster, Jörg Libuda.Fatty Acid Monolayers on Randomly Nanostructured Inorganic Surfaces: Interplay of Wettability, Chemistry, and Topography. Mathieu Beauvais, Irma Liascukiene, Alain M.Intermolecular Hydrogen Bonding between Poly (PHB) and Pseudoboehmite and Its Effect on Crystallization of PHB. Changhao Liu, Meng Jia, Jing Qu, Isao Noda, D.ACS Applied Materials & Interfaces 2021, 13 Spindle-Shaped Surface Microstructure Inspired by Directional Water Collection Biosystems to Enhance Interfacial Wetting and Bonding Strength. ACS Applied Materials & Interfaces 2022, 14 Lattice Strain and Surface Activity of Ternary Nanoalloys under the Propane Oxidation Condition. Leff, Han-Wen Cheng, Shiyao Shan, Shan Wang, Richard Robinson, Dominic Caracciolo, Alex Langrock, David M. Haval Kareem, Yazan Maswadeh, Zhi-Peng Wu, Asher C.This article is cited by 68 publications. The bonding of the ester groups with the oxide surfaces was found to be not stable in the presence of water and also not in the presence of a compound capable of chemisorption with the aluminum oxide surface.
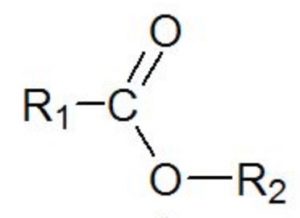
ESTER FUNCTIONAL GROUP FREE
The two compounds showed differences in the free to bonded ν(C O) infrared peak shift, indicating differences in bonding strength with the oxide surface between the two types of molecules. However, the oxides showed differences in the amount of molecules bonded to the oxide surface, and a clear relation was observed with the hydroxyl concentration present on the oxide surface, which was determined from XPS measurements. For all oxides, the ester groups showed the same ν(C O) carbonyl stretching vibration after adsorption, indicating very similar bonding occurs. The ester groups were found to show hydrogen bonding with hydroxyls on the oxide surfaces through their carbonyl oxygens. The extent by which these bonded ester molecules resisted disbondment in water or substitution by molecules capable of chemisorption was also investigated. The purpose of the investigation was to find out what type of ester−oxide bond is formed and whether this is influenced by changes in the composition and chemistry of the oxide. The different oxide layers were made by giving typical surface treatments to the aluminum substrate. The bonding of two types of ester group-containing molecules with a set of different oxide layers on aluminum has been investigated using infrared reflection absorption spectroscopy.


 0 kommentar(er)
0 kommentar(er)
